Role of Lipids and Divalent Cations in Membrane Fusion Mediated by the Heptad Repeat Domain 1 of Mitofusin
Vlieghe A, Niort K, Fumat H, Guigner J-M, Cohen MM, Tareste D.
Biomolecules. 2023; 13(9):1341. https://doi.org/10.3390/biom13091341
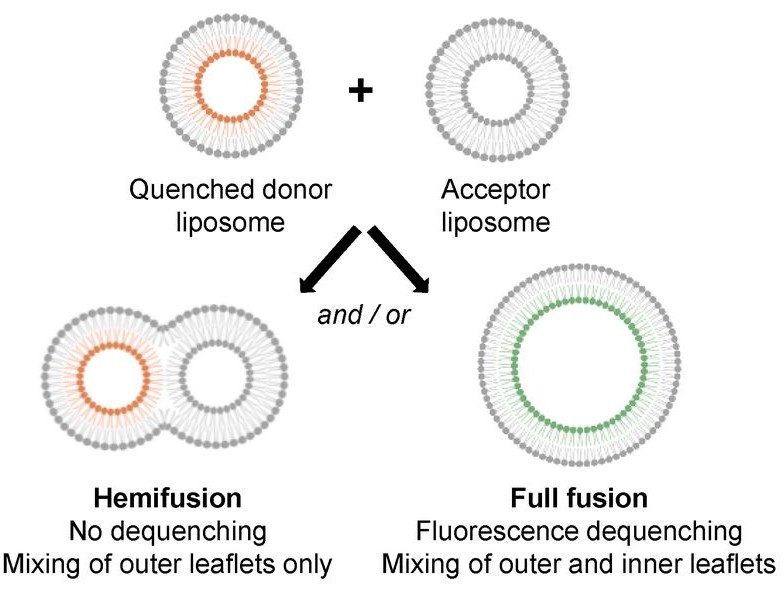
Mitochondria are highly dynamic organelles that constantly undergo fusion and fission events to maintain their shape, distribution and cellular function. Mitofusin 1 and 2 proteins are two dynamin-like GTPases involved in the fusion of outer mitochondrial membranes (OMM). Mitofusins are anchored to the OMM through their transmembrane domain and possess two heptad repeat domains (HR1 and HR2) in addition to their N-terminal GTPase domain. The HR1 domain was found to induce fusion via its amphipathic helix, which interacts with the lipid bilayer structure. The lipid composition of mitochondrial membranes can also impact fusion. However, the precise mode of action of lipids in mitochondrial fusion is not fully understood. In this study, we examined the role of the mitochondrial lipids phosphatidylethanolamine (PE), cardiolipin (CL) and phosphatidic acid (PA) in membrane fusion induced by the HR1 domain, both in the presence and absence of divalent cations (Ca2+ or Mg2+). Our results showed that PE, as well as PA in the presence of Ca2+, effectively stimulated HR1-mediated fusion, while CL had a slight inhibitory effect. By considering the biophysical properties of these lipids in the absence or presence of divalent cations, we inferred that the interplay between divalent cations and specific cone-shaped lipids creates regions with packing defects in the membrane, which provides a favorable environment for the amphipathic helix of HR1 to bind to the membrane and initiate fusion.