“Pat1 coordinates the surveillance of No-Go mRNAs and the fate of their nascent peptides in the context of ribosome-dependent quality control in Saccharomyces cerevisiae”
The defense will take place on Friday, September 26, 2025, at 2:00 PM, in the Edmond de Rothschild Library at the Institut de Biologie Physico-Chimique (IBPC), located at 13 rue Pierre et Marie Curie, 75005 Paris.
Abstract:
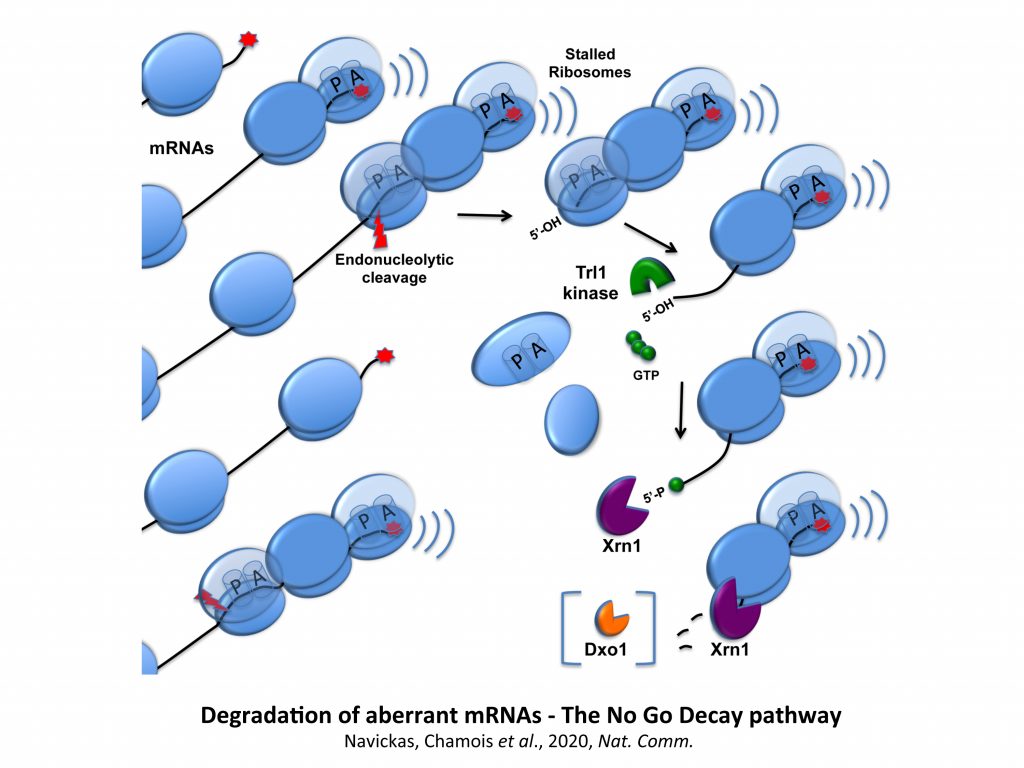
Accumulation of impaired proteins presents a critical challenge for cells, and control systems have evolved to minimize their detrimental effects. Cells have developed mechanisms to address ribosomal stalls caused by problematic translation. mRNAs with ribosomes stalled within the coding sequence are primarily degraded through 5’-decapping and 5’-3’ exonucleolytic processing by the 5’- 3’ exoribonuclease Xrn1. These stalled ribosomes are dissociated by the Ribosome Quality Triggering (RQT) complex, and it is established that a 60S ribosomal subunit is released, still associated with the arrest product (AP), and detected by the Ribosome Quality Control (RQC) complex. The ubiquitinated AP is subsequently directed to the proteasome. How peptide and RNA degradation processes are coordinated remains unclear. It has been shown that ribosomal stalls can be detected by the endonuclease Cue2, leading to mRNA cleavage before dissociation, though this is considered a secondary failsafe mechanism. Therefore, the potential interconnection between peptide degradation and mRNA decay remains to be elucidated. Our findings provide evidence that the decapping activator Pat1 plays a crucial role in orchestrating ribosome-associated quality control. Pat1 is present on problematic mRNAs that have already been targeted for decapping and ensures efficient degradation of the AP. We demonstrate that specific mutants with impaired mRNA decay retain their ability to promote AP degradation, indicating that RNA and AP degradation can be decoupled. Based on our findings, we propose that Pat1 ensures the degradation of faulty mRNAs before stalled ribosomes dissociate, thereby preventing additional rounds of translation, while also actively participating in the degradation of APs.